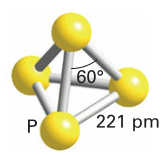
The strength of pi bonds in period 3 elements is lower than those in period 2, making P less favourable than N2. Concentrations of P2 only become appreciable around 800 degrees. Phosphorus at room temperature is usually white phosphorus (tetrahedral units):
If this is kept at 300 degrees in an inert atmosphere for several days, red phosphorus is obtained It has a confusing structure which isn't fully described here. But one advantage is that it does not ignite readily in air:
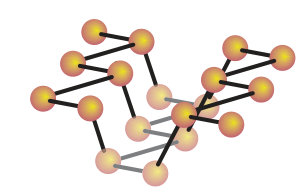
If heated under high pressure, black phosphorus is formed. This consists pluckered layers of 3 co-ordinate atoms:

No comments:
Post a Comment