The number of spin states a nucleus may adopt is determined by its nuclear spin quantum number, I. There are 2I + 1 allowed spin states, with integral differences ranging from +I to -I.
For example, Cl35 has I = 3/2. The range is from +I to -I, so from +3/2 to -3/2. Using integral differences, the allowed spin states are therefor 3/2, 1/2, -1/2, -3/2.
H1 has an I = 1/2, and can adopt a spin state of +1/2 or -1/2, since a single integral difference bridges the gap from +I to -I.
A spinning charge generates a magnetic field of it's own, which points in different directions depending on the direction it spins in. This means the two states do not have equivalent energy when placed inside a magnetic field, since the magnetic fields generated by spinning nuclei could be aligned with the external field, or opposed.
One analogy used is a compass on earth with its needle pointing south. It will spontaneously flip to north if it can do so. To make the analogy better, imagine the energy of the compass is quantitized (for example, it can only point north or south with no intermediates). Also imagine that flipping from south to north will give off one single photon which is equal to the energy difference, and that it can absorb a proton to flip back to south.
.pdf+-+SumatraPDF_2012-11-09_02-23-49.png) |
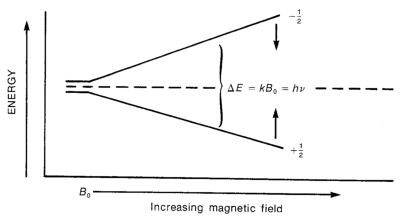
| Splitting of the energy levels of a hydrogen nucleus spin states, as a magnetic force is applied |
Here is the splitting of a Cl35 nucleus:
Note the alignment diagram on the right. Using the compass analogy, it is like we are now given the choice to point northeast and southeast, in additional to north and south.
Here are two extra energy diagrams showing H1 in a magnetic field.
The relationship between field strength and energy difference is an important equation to learn:
h = Planck's constant
v = Frequency of light
B0= Strength of magnetic field (though some people just use B)
γ = Magnetogyric ratio. This is a constant which is dependent on type of element and isotope being studied.
.pdf+-+SumatraPDF_2012-11-09_02-27-22.png)
.pdf+-+SumatraPDF_2012-11-09_02-32-48.png)
.pdf+-+SumatraPDF_2012-11-09_02-32-57.png)
.pdf+-+SumatraPDF_2012-11-09_02-35-58.png)
No comments:
Post a Comment