1. The low energy of the Si-O-Si bending vibration
While these bonds are normally zig-zagged, the planar form is stabilized by negative hyperconjugation, from the pi of oxygen to backlobe of the sigma* Si-Ch3 orbital.
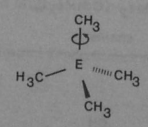
2. Low barriers of conformational changes around the Si-C bond:
Which is because unlike the C-CH3 bonds, the diffuse Si orbitals cannot stabilize certain confirmations.
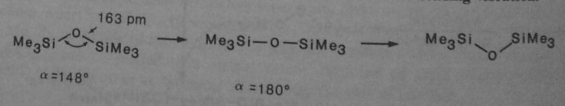
No comments:
Post a Comment