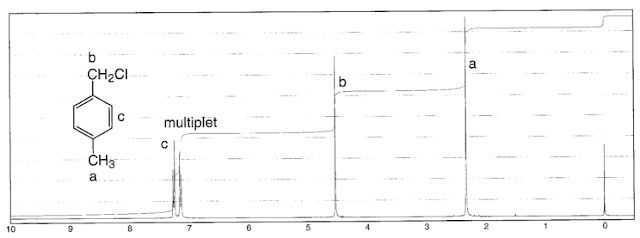
.pdf+-+SumatraPDF_2012-12-22_01-32-38.png) |
| α-chloro-p-xylene - Note the deshielded methyl group, from e- donation into the ring |
Aromatic protons: 6.5 - 8 ppm. These are easily identified since very few environments absorb in this region. A highly deshielded vinyl proton could be found here, but it is rare.
Benzylic protons: 2.3 - 2.7 ppm
The largest shifts are found when electron withdrawing groups such as NO2 are attached, deshielding the protons in the ring. The reverse is true for electron-donating groups like MeO.
Aromatic protons experience coupling right across the ring, but the clear differences in J values often allow substitution patterns to be determined by measuring them - described in a future post.
.pdf+-+SumatraPDF_2012-12-22_01-19-03.png)
What is the splitting pattern of methylene and methyl protons
ReplyDelete