.pdf+-+SumatraPDF_2012-12-19_00-53-12.png) |
| Butylamine |
.pdf+-+SumatraPDF_2012-12-19_00-53-22.png) |
| Dibutylamine |
.pdf+-+SumatraPDF_2012-12-19_00-53-35.png) |
| Tributylamine |
.pdf+-+SumatraPDF_2012-12-19_00-53-45.png) |
| N-methylaniline |
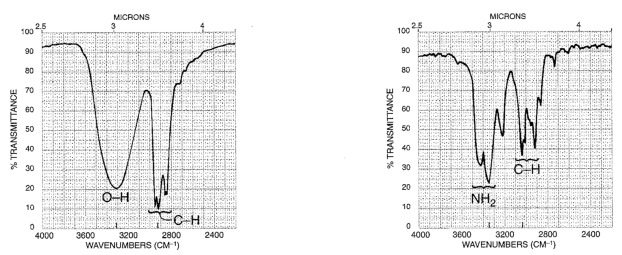
N-H stretch: At 3500-3300. This is split into two bands for a primary, one for a secondary, and is nonexistent for a tertiary amine. The intensity can be small for aliphatics, often vanishingly small for secondary aliphatic amines. For aromatics the stretch can be quite large.
The higher frequency band is from a symmetric vibration, the lower from asymmetric vibration.
In dilute solutions (non hydrogen-bonded) the peaks are shifted higher.
N-H in-plane-bend: A broad peak at 1640-1560 for primary amines. Secondary amines absorb near 1500. Can overlap a C=C peak.
N-H oop bend: Sometimes observed near 800, seen more easily in aliphatic amines.
C-N stretch: Occurs at 1350-1000, varying intensity. 1250-1000 for aliphatics, 1350-1250 for aromatics. The higher frequency in aromatics is from resonance increasing double-bond character between the ring and attached nitrogen.
For a neat liquid, the N-H peak is typically weaker and sharper than O-H:
No comments:
Post a Comment