- Molecules at room temperature are constantly colliding with other molecules, bouncing away due to charge-charge repulsion
- Not all collisions lead to a reaction
- Whether a reaction happens depends on whether the molecules are given enough energy (the activation energy) to overcome charge-charge repulsion.
- It can also depend on the angle of attack. Reactions depend on the overlap of orbitals, which are usually not a uniform sphere.
- Species can be attracted by charges and dipoles
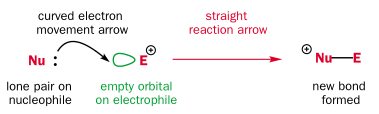
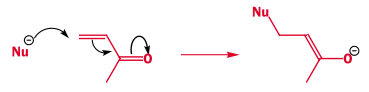
- Electron flow is the key to reactivity and making new bonds. This is a typically a lone pair donor (the nucleophile) donating a pair of electrons to the empty orbital of a lone pair acceptor (the electrophile). This transfer is shown by curly arrows.
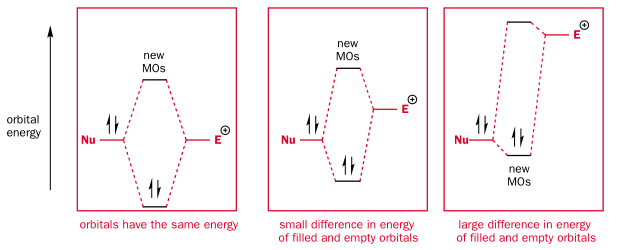
-When the two orbitals overlap, they produce a new high and low energy MO. The closer the energy levels of overlapping orbitals, the higher the energy gain:
.
Only the HOMO of the nucleophile (nearly always a lone pair) is likely to be close in energy to the unoccupied orbital of the electrophile. So overlap of other non-HOMO/non-lone pair orbitals with the empty orbital is neglected.
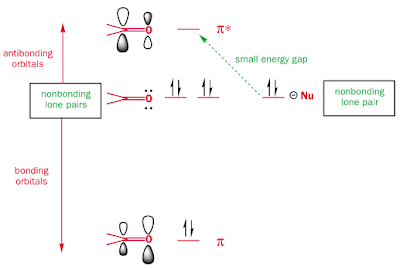
- The acceptor orbital is often an antibonding MO orbital. Pi* is common, since it is usually unoccupied, and is lower in energy than sigma* MOs. The most common example of this is nucleophillic attack of a carbonyl group.
The carbon is the atom attacked because it has the larger lobe on the antibonding orbital. It also has a slight positive dipole.
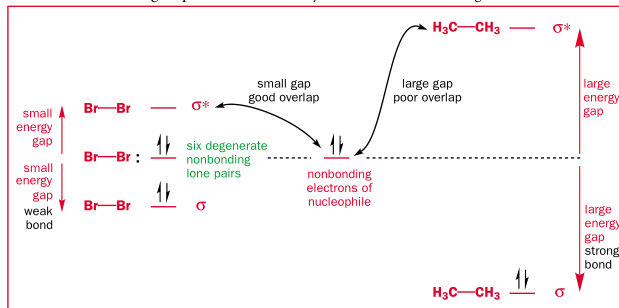
An acid can be considered a HOMO -> sigma* reaction. Especially when a proton is bonded to a more electronegative atom, which brings down the energy of the sigma* orbital.
The dipole produced by an electronegative atom also increases the chance of nucleophillic attack. But the lowered sigma* is much more important. Many sigma bonds are electrophillic even with a negligible or no dipole, such as C-I and Br2.
Bond strength also depends on other factors then how close the overlapping orbitals are. For example, the Br-Br bond is much weaker than the C-C bond, so it has a lower-lying sigma*, which is why only Br2 is considered an electrophile.
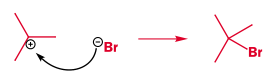
- Pi bonds can act as a nucleophile:
Which in this case is followed by nucleophillic attack of a bromine lone pair onto the empty carbocation p orbital:
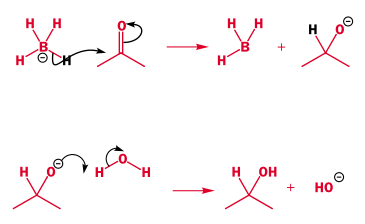
- In some cases, such as borohydrides, a weak sigma bond can be the nucleophile. Below is a sigma -> pi* reaction:
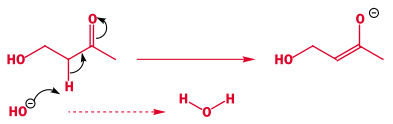
- Electrons moving within molecules are also shown as curly arrows





No comments:
Post a Comment