SH2 is a flammable poisonous gas with the odor of rotten eggs - produced by the anaerobic breakdown of proteins by bacteria.
Using the acidity constants and initial concentrations of the acid, many questions will ask for the concentration of a particular ion. These usually require clever approximations. Example:
It is interesting that this approximation gives an answer independent of the initial acid concentration.
Obviously we can change the concentration of a specific species by changing pH, which changes [H3O+] in the acidity constant equations. In distribution diagrams, the fraction of a solute present as a specific species, is shown as a function of pH.
For example, consider the triprotic acid H3PO4. The fraction of molecules present as intact H3PO4 is:
And here is the distribution diagram:


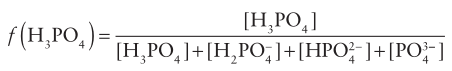

As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. Acid Yellow 72
ReplyDelete