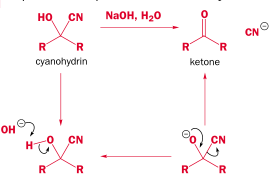
There are two problems with the above method. First, the final step is very slow and has a tendency to reverse:
The solution is to speed up the final step by addition of an acid:
A second problem is that hydrogen cyanide is a deadly gas, which has to be bubbled through water to dissolve. This was the gas used in the holocaust, or to execute prisoners in parts of the united states.
The solution is to use a solid salt containing the cyanide ion instead:
Solid cyanide is a white powder which is just as deadly as HCN if you consume it. Cyanide salts are often in the suicide pills used in spy movies/novels, or as poison in detective stories. But in the lab, a deadly solid is a lot easier to handle than a deadly gas.
Of course, small amounts of hydrogen cyanide will bubble out anyway as the reaction progresses, due to the competing reaction:
So the reaction should still be completed in a fume hood.
By using sodium cyanide, we have less acidic protons in the solution, which are necessary for the final step. But this is more than made up for by the HCl from the first improvement.
Just as acid pushes forward the final slow step - a base will push it back:
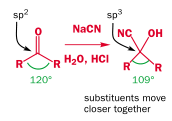
Overall it is an equilibrium reaction. This more favors the product for aldehydes than ketones, because of steric strain from the R groups:
Many reactions of this type will use both HCN and NaCN at once. It obviously works, but I don't know why this is common.






This comment has been removed by the author.
ReplyDelete