 |
| 1s |
 |
| 2s |
 |
| 2p |
The wave function squared, or ψ2, represents the probability of finding that electron in that point in space. Which look like this:
Wave functions can have positive and negative phases. Overlap of opposite phases will cancel out. Physicists sometimes use analogies with macroscopic waves such as water waves, where peaks and troughs will reinforce their own kind, but will cancel eachother out.
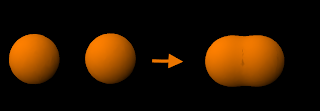
Constructive (bonding) overlap of 1s orbitals:
Destructive (antibonding) overlap of 1s orbitals:
 |
| A node is a point of 0% probability of finding the electron |
I don't think there exists a satisfying explanation why electrons act like this - why they act like waves with different phases and so on. All we know is that there is a mathematical model which describes them - called a wavefunction because it's similar to the mathematical models describing classic waves - and that it fits well with experiment.
Perhaps when we use computers to upgrade our brains it will be as simple as "a bucket of sand and bucket-shaped hole will cancel out" is to us - and we'd be able to rest knowing that the universe acts in a nice and appropriate way.
In drawings or 3d models, phases are usually shown as shades, different colors, or + and - symbols. Bonding orbitals are considered "in-phase overlap":
Anti-bonding orbitals are considered to be "out of phase overlap", producing these abominations:







No comments:
Post a Comment