Pages
▼
Group 14 B
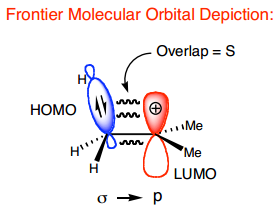
Alkyne analogues have decreasing bond order down the group. C is a triple bond sp hybrid, Si/Ge/Sn are double bond sp2 hybrids, and Pb forms single bonds with no hybridisation. The E-E distance increases down the group. The degree of bending also increases - alkynes are linear, Pb-Pb-R is at a 90 degree angle.
The decreasing willingness to hybridize is expressed in Bent's rule:
Atomic s character tends to concentrate in orbitals that are directed toward electropositive groups and atomic p character tends to concentrate in orbitals that are directed toward electronegative groups
Polarity when bonded to carbon increases down the group, which is associated with an increase in p character and a decrease in s character of the bonding orbitals.
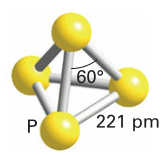
Phosphorus
The strength of pi bonds in period 3 elements is lower than those in period 2, making P less favourable than N2. Concentrations of P2 only become appreciable around 800 degrees. Phosphorus at room temperature is usually white phosphorus (tetrahedral units):
If this is kept at 300 degrees in an inert atmosphere for several days, red phosphorus is obtained It has a confusing structure which isn't fully described here. But one advantage is that it does not ignite readily in air:
If heated under high pressure, black phosphorus is formed. This consists pluckered layers of 3 co-ordinate atoms:
If this is kept at 300 degrees in an inert atmosphere for several days, red phosphorus is obtained It has a confusing structure which isn't fully described here. But one advantage is that it does not ignite readily in air:
If heated under high pressure, black phosphorus is formed. This consists pluckered layers of 3 co-ordinate atoms:
Organosilicons 2
The high felixibility of silicones can be traced back to two things.
1. The low energy of the Si-O-Si bending vibration
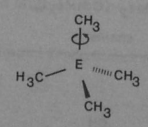
2. Low barriers of conformational changes around the Si-C bond:
1. The low energy of the Si-O-Si bending vibration
While these bonds are normally zig-zagged, the planar form is stabilized by negative hyperconjugation, from the pi of oxygen to backlobe of the sigma* Si-Ch3 orbital.
2. Low barriers of conformational changes around the Si-C bond:
Which is because unlike the C-CH3 bonds, the diffuse Si orbitals cannot stabilize certain confirmations.
Organotin and organolead compounds
Organotin compounds are used in antifouling agents, such as on ships and wood preservative. They are also added to PVC to prevent being degraded by the atmosphere.
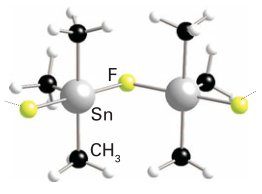
Halide derivatives, R3SnX, often contain Sn-X-Sn bridges and form chains. Bulky R groups can affect this. For example, (SnFMe3)n has the Sn-F-Sn backbone in a zig-zag arrangement.
Alkyltin compounds can be prepared from tetrachlorotin, using Grignard's reagent:
They can also be prepared by metathesis between chlorotin and alkyl aluminium:
Halide derivatives, R3SnX, often contain Sn-X-Sn bridges and form chains. Bulky R groups can affect this. For example, (SnFMe3)n has the Sn-F-Sn backbone in a zig-zag arrangement.
Tetraethyl lead produced on a huge scale as an anti-knocking agent in petrol. It can be made using Grignard's reagent. This time producing a mole of Pb:
Like alykl silicon, alkyl lead can also be made from alkyl lithiums:
Lead can also contain bridging halide atoms, if the R groups are not too bulky.
Organosilicon compounds
The carbon-silicon bond is strong and its compounds are fairly stable. They can be prepared with silicon chloride and alkyl lithium:
The number of chlorides replaced depends on the ratio of reactants.
Converting silicon metal to methylchlorosilane can be done using chloromethane and a bit of copper. This is called the Rochow process:
The number of chlorides replaced depends on the ratio of reactants.
Converting silicon metal to methylchlorosilane can be done using chloromethane and a bit of copper. This is called the Rochow process:
n can take the value of 1 - 3.
Chloromethane can then be hydrolysed to trimethylsilanol:
Which condenses to a dimer:
With Me2SiCl2, this forms chains or rings called silicones - molecules with a Si-O-Si backbone. The Si-O bond is much stronger than Si-Si, and is similar in strength to a C-C bond, so it allows very long chains.
Larger numbers of chlorides, when hydrolysed, produce branching and cross-linking:
Branching between methyl groups can also be achieved. This is done by using Benzoyl Peroxide, a radical initiator:
Liquid silicones are more stable than hydrocarbons, and have a much lower change of viscosity with temperature. Thus they are used in brake fluid.
Silicones are hydrophobic, so they are used in water-proof sprays for shoes.
Low-mass silicones are used in shampoo, conditioner, shaving foam, hair gel, and toothpaste, to impart a silky feel.
Larger-mass greases, oils and resins are used for sealants, lubricants, varnishes, and synthetic rubbers
Group 14
Carbon and Silicon are non-metals, Germanium a metalloid and lead a metal. The metallic character down a group is associated with:
1. Lower ionization energy, making them more willing to form cations and lose electrons into a "sea".
2. More diffuse shells, meaning less directional bonding.
Carbon and silicon have a high affinity for hard anions such as O2- and F-. The heavier group 14 elements such as lead prefer soft anions such as I- and S2-. Hardness refers to small radii, high oxidation states and low polarizability.
All group 14 elements form tetravalent hydrides (EH4, catenation (bonds between the same element) decreases down the group. This is associated with decreasing E-E bond entropy.
The largest silane (SinHn+2 chain that can form is heptasilane. Silicon's larger number of electrons means more intermolecular force. So while propane is a gas at room temperature trisilane boils at 53 degrees.
Something called the inert-pair effect increases down the group, which results in a tendency to form +2 rather then +4.
1. Lower ionization energy, making them more willing to form cations and lose electrons into a "sea".
2. More diffuse shells, meaning less directional bonding.
Carbon and silicon have a high affinity for hard anions such as O2- and F-. The heavier group 14 elements such as lead prefer soft anions such as I- and S2-. Hardness refers to small radii, high oxidation states and low polarizability.
All group 14 elements form tetravalent hydrides (EH4, catenation (bonds between the same element) decreases down the group. This is associated with decreasing E-E bond entropy.
The largest silane (SinHn+2 chain that can form is heptasilane. Silicon's larger number of electrons means more intermolecular force. So while propane is a gas at room temperature trisilane boils at 53 degrees.
Something called the inert-pair effect increases down the group, which results in a tendency to form +2 rather then +4.
NMR 4
One of the most deshielded protons found in organic molecules is an aldehyde protons, at shifts of 9 to 10 ppm:
If the solution is concentrated enough for hydrogen bonding to take place, it will deshield these hydrogens even further:
The resonance is 1 or 2 ppm higher for carboxylic acids, due to this resonance form:
NMR 3
Protons found in chemically equivalent environments usually have the same chemical shift. Below are some examples. These use spectra predicted by a computer program, so the actual location of the shift is not very reliable.
One environment:

One environment:
Two environments:
Three environments:

In H1 NMR, the area under each peak is proportional to the number of protons generating that peak. NMR machines typically integrate (find the area under) the curves automatically, which is often represented as an additional line. The example of benzyl acetate is below:
.pdf+-+SumatraPDF_2012-11-09_18-11-26.png) |
| Benzyl acetate NMR |
You can tell from the molecular formula that there are three H environments (assume the aromatic protons are in resonance), populated in a 5:3:2 ratio. The integrals on the spectra reflect this.
Note that integrals give relative numbers, not absolute numbers. There needs to be more than one peak for the integral to be useful. Integrals also have quite a large error range, of about 10%. Forgetting this error range is a common mistake on spectroscopy exams - where students find the correct formula but believe it has the wrong number of hydrogens.
The shift of each peak also tells us about the environment of those protons, for example, aromatic protons are typically found between 7 - 8 ppm, and methyl groups between 0 - 2 ppm. You can find good charts for these in the "spectroscopy data" link at the top of the blog.
You should also remember that deshielding shifts peaks to the left. Electronegative atoms like oxygen will inductively deshield nearby atoms. In the molecule above, the CH2 is next to an oxygen, while the CH3 is two bonds away, hence the CH2 is more deshielded. The even higher amount of deshieldling for the aromatic molecules are explained by magnetic anisotropy, described in a future post.
NMR 2
When a nucleus is shielded by electrons, these circulate to partially counteract an external magnetic field. This is circulation is called a diamagnetic current. The shielding effect produced by this current is called diamagnetic shielding.
So heavily shield protons experience a reduced magnetic field, hence a reduced energy gap, hence they absorb light at a lower frequency. Deshielding therefore increases the frequency of absorbed radiation.
Rather then measuring frequency of absorption directly, NMR usually measures absorption relative a reference compound. The most common reference is tetramethylsilane:
So heavily shield protons experience a reduced magnetic field, hence a reduced energy gap, hence they absorb light at a lower frequency. Deshielding therefore increases the frequency of absorbed radiation.
Rather then measuring frequency of absorption directly, NMR usually measures absorption relative a reference compound. The most common reference is tetramethylsilane:
This is chosen because its protons are more shielded than usual. This prevents overlap with protons on most organic molecules.
The frequency which a proton absorbs at also depends on the magnetic field strength of the NMR machine, which varies according to research budgets, since strong magnets increase the resolution, but are more expensive. To allow easy comparison of specta, the field-independent frequency can be measured using chemical shift notation (δ).
The (shift in Hz) part refers to the shift relative to tetramethylsilane or another standard. Since this is divided by MHz, then chemical shift could also be described as "shift from tetramethylsilane in parts per million of the spectrometer's operating frequency".
Guide to NMR
NMR can only be conducted on atomic nuclei which have spins. These have either an odd mass number, odd atomic number, or both. The two introduced during A-level chemistry were H1 and C13, undergrad level introduces others.
The number of spin states a nucleus may adopt is determined by its nuclear spin quantum number, I. There are 2I + 1 allowed spin states, with integral differences ranging from +I to -I.
For example, Cl35 has I = 3/2. The range is from +I to -I, so from +3/2 to -3/2. Using integral differences, the allowed spin states are therefor 3/2, 1/2, -1/2, -3/2.
H1 has an I = 1/2, and can adopt a spin state of +1/2 or -1/2, since a single integral difference bridges the gap from +I to -I.
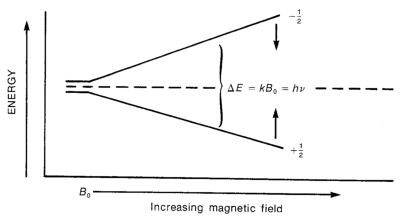
A spinning charge generates a magnetic field of it's own, which points in different directions depending on the direction it spins in. This means the two states do not have equivalent energy when placed inside a magnetic field, since the magnetic fields generated by spinning nuclei could be aligned with the external field, or opposed.
One analogy used is a compass on earth with its needle pointing south. It will spontaneously flip to north if it can do so. To make the analogy better, imagine the energy of the compass is quantitized (for example, it can only point north or south with no intermediates). Also imagine that flipping from south to north will give off one single photon which is equal to the energy difference, and that it can absorb a proton to flip back to south.
Just so the graph above doesn't deceive you: the energy levels change linearly with magnetic field strength they do not level off.
Here is the splitting of a Cl35 nucleus:
The number of spin states a nucleus may adopt is determined by its nuclear spin quantum number, I. There are 2I + 1 allowed spin states, with integral differences ranging from +I to -I.
For example, Cl35 has I = 3/2. The range is from +I to -I, so from +3/2 to -3/2. Using integral differences, the allowed spin states are therefor 3/2, 1/2, -1/2, -3/2.
H1 has an I = 1/2, and can adopt a spin state of +1/2 or -1/2, since a single integral difference bridges the gap from +I to -I.
A spinning charge generates a magnetic field of it's own, which points in different directions depending on the direction it spins in. This means the two states do not have equivalent energy when placed inside a magnetic field, since the magnetic fields generated by spinning nuclei could be aligned with the external field, or opposed.
One analogy used is a compass on earth with its needle pointing south. It will spontaneously flip to north if it can do so. To make the analogy better, imagine the energy of the compass is quantitized (for example, it can only point north or south with no intermediates). Also imagine that flipping from south to north will give off one single photon which is equal to the energy difference, and that it can absorb a proton to flip back to south.
.pdf+-+SumatraPDF_2012-11-09_02-23-49.png) |
| Splitting of the energy levels of a hydrogen nucleus spin states, as a magnetic force is applied |
Here is the splitting of a Cl35 nucleus:
Note the alignment diagram on the right. Using the compass analogy, it is like we are now given the choice to point northeast and southeast, in additional to north and south.
Here are two extra energy diagrams showing H1 in a magnetic field.
The relationship between field strength and energy difference is an important equation to learn:
h = Planck's constant
v = Frequency of light
B0= Strength of magnetic field (though some people just use B)
γ = Magnetogyric ratio. This is a constant which is dependent on type of element and isotope being studied.

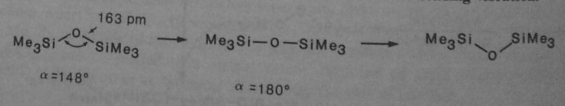







.pdf+-+SumatraPDF_2012-11-10_04-31-47.png)
.pdf+-+SumatraPDF_2012-11-10_04-32-53.png)
.pdf+-+SumatraPDF_2012-11-10_04-35-33.png)




.pdf+-+SumatraPDF_2012-11-09_17-31-26.png)
.pdf+-+SumatraPDF_2012-11-09_04-19-59.png)

.pdf+-+SumatraPDF_2012-11-09_04-32-04.png)
.pdf+-+SumatraPDF_2012-11-09_02-27-22.png)
.pdf+-+SumatraPDF_2012-11-09_02-32-48.png)
.pdf+-+SumatraPDF_2012-11-09_02-32-57.png)
.pdf+-+SumatraPDF_2012-11-09_02-35-58.png)