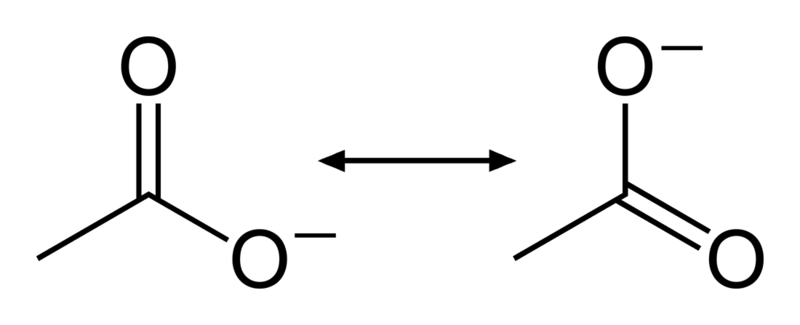
A double-bond and single-bond have different lengths. The two oxygen bond lengths in this anion are detected experimentally as halfway between the lengths of single and double bonds. So the true nature of a molecule is a blend of their resonance structures.
A structure with multiple resonances is also lower in energy then any single contributing structure. So the negative energy of the above anion is even lower then summing the individual bonds would suggest.
Resonance structures are usually depicted as having the same energy as each other. Structures slightly higher in energy can also contribute, but won't do so as much. Chemists usually have some intuition about what are the stable and therefore most contributing resonance structures. Low-energy structures usually have:
Atoms obeying the octet rule
Negative charge located on electronegative atoms, positive charge on the electropositive atoms
More bonds
No charges
If charge is necessary, have it spread out across the molecule
Consider these negligible resonances for the acetate ion.
This one has both more charge and less bonds, making it too unstable to have a significant contribution.
This has an extra bond, but the negative charge is on an less electronegative element, and most importantly it violates the octet rule for carbon. We never usually see carbon break the octet rule, so this structure would immediately look wrong to a chemist. But it might work if another atom was in place of carbon.
Also consider Furan:
Positive charge on the oxygen is not going to be very stable, making the first structure the main contributor. But we can expect experiments to show all the single bonds as having slightly double-bond character.


No comments:
Post a Comment